How Do Soda Companies Get the CO2 into Cans?
Ever wonder how soda companies get that bubbly fizz into your favorite canned drinks? What if the process is more scientific than you think, involving specialized equipment and careful chemical reactions?
Soda companies use a process called carbonation to get CO2 into cans. This involves chilling the liquid, adding CO2 under high pressure, and sealing the can. The high pressure forces the CO2 to dissolve in the liquid, and the sealed can prevents the CO2 from escaping, creating the characteristic fizz when opened.

Want to uncover the secrets behind the perfect fizz in your soda? Keep reading to find out how soda companies make it happen using these innovative CO2 generators.
How do soda companies get CO2?
Are you curious about how soda companies obtain the CO2 they need for their drinks? Is it all purchased from external suppliers, or do some companies produce their own?
Soda companies can get CO2 in a few ways. Some purchase it from industrial gas suppliers, while others use food-grade carbon dioxide generators to produce their own. These generators use chemical reactions in a closed system to ensure purity and safety.

Methods of Obtaining CO2: Dive Deeper
Purchasing from Suppliers
- Industrial Gas Companies: Many soda companies buy CO2 from specialized industrial gas suppliers.
- Purity Standards: These suppliers ensure the CO2 meets stringent food-grade standards.
- Transportation: The CO2 is transported in specialized tanks to the soda production facilities.
On-Site Generation
- CO2 Generators: Some companies use on-site CO2 generators to produce their own CO2.
- Chemical Reactions: These generators use controlled chemical reactions to create CO2.
- Closed Systems: The reactions occur in closed systems to prevent contamination and ensure purity.
Comparing the Options
| Option | Advantages | Disadvantages |
|---|---|---|
| Purchasing from Suppliers | Reliable supply, guaranteed purity, no need for on-site equipment | Dependence on external suppliers, transportation costs, potential supply chain issues |
| On-Site Generation | Control over supply, potentially lower costs, reduced transportation needs | Initial investment in equipment, maintenance requirements, expertise needed |
I once spoke with an engineer at a soda bottling plant who explained their decision to invest in an on-site CO2 generator. He said it gave them more control over their supply and ultimately reduced their costs in the long run. However, he also mentioned the initial investment was significant.
How do they get soda into soda cans?
Are you wondering about the process of filling soda into cans while maintaining the crucial carbonation? How do soda companies ensure efficiency and precision in this process?
Soda water companies use professional three in one filling machines to quickly seal while completing the filling process, ensuring minimal carbon dioxide spillage

The Filling Process: Dive Deeper
Key Steps
- Can Cleaning: Empty cans are cleaned and sterilized to remove any contaminants.
- Filling: The cans are filled with chilled, carbonated soda using precise filling nozzles.
- Sealing: The cans are immediately sealed to prevent CO2 from escaping and maintain carbonation.
- Quality Control: Filled and sealed cans are inspected to ensure proper fill levels and seal integrity.
Specialized Filling Machines
- Isobaric Fillers: These fillers maintain constant pressure during the filling process to prevent CO2 from escaping.
- Counter-Pressure Fillers: These fillers use counter-pressure to minimize agitation and maintain carbonation.
Automation and Efficiency
- High Speed: Modern filling machines can fill hundreds or even thousands of cans per minute.
- Precision: Automated systems ensure accurate fill levels, reducing waste and maintaining consistent product quality.
- Sterile Environment: The filling process takes place in a controlled, sterile environment to prevent contamination.
I’ve seen some of our EQS filling machines being used and I'm always impressed by how quickly and efficiently they can fill thousands of cans while maintaining the perfect level of carbonation.
Where does beverage grade CO2 come from?
Are you curious about the specific origins and processes that ensure the safety and quality of beverage-grade CO2, especially when it's generated on-site?
Beverage-grade CO2 can come from various sources, including natural sources, industrial processes, and dedicated CO2 generators. When generated on-site, it undergoes rigorous purification and quality control to meet the stringent standards required for use in beverages.

The Journey of Beverage-Grade CO2: Dive Deeper
Natural Sources
- Natural CO2 Wells: In some regions, CO2 is extracted from underground wells. This CO2 is naturally occurring and can be purified for beverage use.
- Volcanic Activity: CO2 can also be captured from volcanic activity, although this is less common due to logistical challenges.
Industrial Processes
- Ammonia Plants: CO2 is a byproduct of ammonia production. This CO2 can be captured and purified for use in beverages.
- Ethanol Production: Similar to ammonia plants, ethanol production also generates CO2 as a byproduct.
On-Site CO2 Generators
- Chemical Reactions: These generators use controlled chemical reactions to produce CO2.
- Purification: The generated CO2 is purified to remove any impurities.
Quality Control
| Purity Standard | Description | Importance |
|---|---|---|
| Food Grade | CO2 must meet food-grade standards, as defined by regulatory agencies. | Ensures it is safe for consumption and does not contain harmful contaminants. |
| Testing | CO2 is tested for impurities such as sulfur compounds, hydrocarbons, and other gases. | Guarantees it will not affect the taste, odor, or safety of the beverage. |
| Certification | CO2 suppliers and generators must be certified and regularly audited to ensure compliance. | Provides assurance that the CO2 meets the required standards and is safe for use in the beverage industry. |
I learned from a beverage factory's quality control team that testing CO2 for purity is extremely important. They use advanced equipment and strict procedures to ensure that only the highest quality CO2 is used in their products.
Where do the bubbles come from in carbonated drinks?
Have you ever wondered how soda companies create that satisfying fizz in carbonated drinks, especially when using CO2 generated on-site?
The bubbles in carbonated drinks come from dissolved carbon dioxide (CO2) gas. During the carbonation process, CO2, whether sourced externally or generated on-site, is forced to dissolve into the liquid under high pressure. When the pressure is released, such as when you open a can or bottle, the CO2 comes out of solution and forms bubbles.
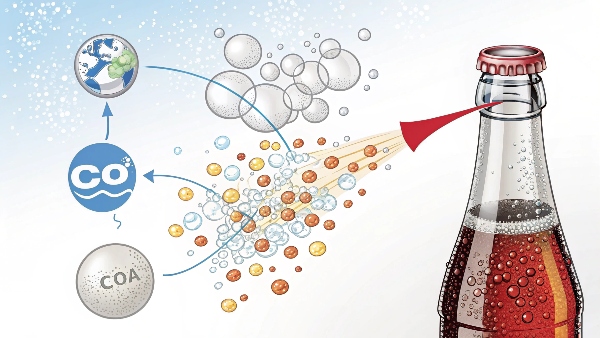
The Science of Bubbles: Dive Deeper
The Carbonation Process
- High Pressure: CO2 is dissolved in the liquid under high pressure, which forces the gas molecules to squeeze between the liquid molecules.
- Low Temperature: Lower temperatures help CO2 dissolve more easily in the liquid.
- Sealed Container: The carbonated liquid is stored in a sealed container to maintain the pressure and keep the CO2 dissolved.
Release of Pressure
- Opening the Container: When you open a can or bottle, the pressure is released, causing the CO2 to come out of solution.
- Bubble Formation: The dissolved CO2 forms bubbles as it escapes from the liquid.
- Fizzing Sensation: These bubbles create the characteristic fizzing sensation that we associate with carbonated drinks.
Factors Affecting Bubbles
| Factor | Effect | Explanation |
|---|---|---|
| Temperature | Warmer drinks lose CO2 faster | CO2 is less soluble in warmer liquids, causing it to escape more quickly. |
| Agitation | Shaking a carbonated drink releases CO2 | Agitation provides nucleation sites for CO2 bubbles to form, accelerating the release of gas. |
| Surface Tension | Drinks with lower surface tension have smaller, more stable bubbles | Lower surface tension allows bubbles to form more easily and persist longer. |
I remember doing a project on carbonation in college, and it was fascinating to learn how different factors affect the fizz. Temperature, pressure, and even the type of liquid all play a role in creating the perfect carbonated beverage.
Conclusion
Whether soda companies purchase CO2 or generate it on-site, the process of getting that CO2 into your can of soda is a carefully controlled and scientific endeavor. Understanding this process can give you a new appreciation for the science behind every sip.
My name is Allen, and I'm an expert in filling machine technology at EQS (eqsfilling.com), a leading liquid packaging solution provider based in China. If you're looking for top-quality filling machines for your production line, feel free to reach out to me at [email protected]. We specialize in providing customizable solutions with cutting-edge technology.






